If we look at a mouse with a deletion of the gene for cytoplasmicG3Pdh we have a reasonable model for elimination of the glycerophosphate shuttle at the level were glycolysis would normally be used to drive reverse electron flow through complex I, to facilitate insulin signalling. These mice are remarkably normal and can swim in deep water, possibly not too happily, for at least 20 minutes with weights on their tails, i.e. they can exercise, at least if necessary to save their lives. They have markedly reduced levels of pyruvate and mildly elevated levels of lactate in muscle tissue compared to control mice.
It's the bottom two lines which I looked at. The lactate to pyruvate ratio reflects the NADH to NAD+ ratio within the cytoplasm, as Krebs puts it:
In a cell [H+] is a constant and K is also a constant (by definition).
So clearly we have a lot more NADH available in the knockout mouse, so more lactate gets formed from pyruvate, which becomes a minor player in the cascade of glycolysis to oxidative phosphorylation allowing lactate to take over. The authors of the mouse paper suggest that the lactate is expelled from the cells and that the Cori cycle, in the liver, is active to deal with the it. I’d prefer to think of lactate as being shunted directly to the mitochondria for use in the TCA.
If you subscribe to the view that the glycerophosphate shuttle is needed to provide NAD+ for glycolysis to proceed you might expect a few problems with glucose processing. There is undoubtedly an accumulation of metabolites upstream of the glycerophosphate shuttle and a depletion of those downstream but glycolysis does proceed. But from my point of view, with no glycerophosphate shuttle, there is nothing to allow the body to facilitate insulin’s drive to self activate using glucose. How do these mice cope?
They cope very well.
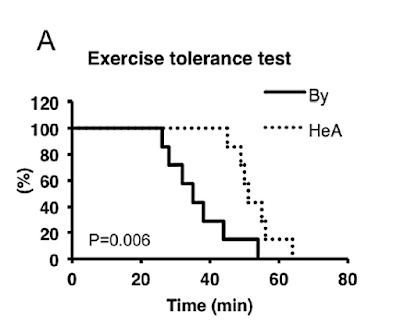
If you feel that tying a weight to the tail of a mouse and dropping it a beaker of water is a bit too crude, there are more sophisticated methods of inducing exercise. Worse than making obese people do cardio at any gym where fat shaming rules. It's possible to run a mouse to utter exhaustion and monitor its respiratory quotient while it runs. So you can see whether it burns predominantly glucose or fat and in what balance. You can also measure exactly how long it can run for, before it collapses at the level of exhaustion where it can no longer avoid an electroshock or two or three. Here are the core findings from sending mice to an electro-gym (the Thumb Tack Hypothesis taken to serious levels):
HeA are the knockout mice, By are the control mice. Knockout mice run harder and for longer than control mice. The rest of the graphs use the same coding, solid line is the control mice, dotted line the knockouts.
Taken from the RQ we can ask whether the knockout mice can oxidise glucose. Yes:
Pretty much as well as the control mice.
Can they oxidise lipid? Yes, somewhat better than can the control mice:
I think it is also worth noting that under marked but non-exhausting exercise that glycogen in the muscle of knockout mice does not fall, it does so in control mice:
These modified mice, which cannot use glycolysis to trigger insulin signalling, have a tendency to have MORE glycogen in their muscles (although p is greater than 0.05) at rest and they deplete it less under sustained near-maximal exercise. I'd guess insulin does signal.
It's also worth noting that blood lactate under the same conditions does not rise in the knockout mice whereas it does in control mice (p less than 0.05, yay!). My assumption is that lactate is being metabolised in the muscles of knockout mice and shunted to the liver for the Cori Cycle in control mice:
So what might be going on in these knockout mice? The requirement for insulin signalling is a modest amount of reverse flow of electrons through complex I, ie the CoQ couple must be reduced. The usual, here absent, technique is the glycerophosphate shuttle. But we can reduce the CoQ couple in other ways. My favourite way is via the oxidation of the FADH2 generated by metabolism of saturated fatty acids. How much FADH2 is needed to replace the glycerophosphate shuttle?
From the graph of lipid oxidation above we can see that knockout mice under exercise are oxidising somewhere around 45mg/kg/min of fat. The control mice are oxidising just over 30mg/kg/min. From the Protons perspective the increased fat oxidation is a requirement for normal insulin signalling and this insulin signal cannot limit fatty acid oxidation until the rate is almost 50% higher in the knockout mice than in those where the glycerophosphate shuttle works. These mice oxidise fat because insulin signalling is not being triggered by glycolysis. It also means that lipid oxidation has to be higher before it can trigger insulin resistance and cellular energy influx limitation.
We don't (as far as I know) have a drug to inhibit cytG3Pdh.
We do have one to inhibit mtG3Pdh, the other half of the glycerophosphate shuttle.
It's called metformin. Does metformin do the same thing as having a cytG3Pdh knockout does? Under exercise? In terms of getting the King Of the Mountains jersey in the Tour de France perhaps?
Possibly so.
Peter
Summary: Metformin blocks glycolysis triggered insulin signalling and cells replace this with FADH2 triggered insulin signalling from fatty acid oxidation (at ETFdh). This results increased fatty acid oxidation and in improved high intensity exercise ability. Oh, and I guess weight loss etc...
Aside. I think I might start sticking the refs from a post in at the end. There are times I can't remember in which post a paper was used, searching my own blog/hard drive might be easier if the author or a keyword are actually present rather than there just being a highlighted text field!!! The blog is getting a bit unwieldy.
Glycerol 3-phosphate dehydrogenase 1 deficiency enhances exercise capacity due to increased lipid oxidation during strenuous exercise
Mouse lacking NAD+-linked glycerol phosphate dehydrogenase has normal pancreatic beta cell function but abnormal metabolite pattern in skeletal muscle
The Redox State of Free Nicotinamide-Adenine Dinucleotide in the Cytoplasm and Mitochondria of Rat Liver
Metformin improves performance in high-intensity exercise, but not anaerobic capacity in healthy male subjects.
Home » Protons (40) Living without the glycerophosphate shuttle
» Protons (40) Living without the glycerophosphate shuttle
Langganan:
Posting Komentar (Atom)







0 Response to "Protons (40) Living without the glycerophosphate shuttle"
Posting Komentar